Sodium hypobromite (NaOBr) PROCEDURE Take 1 ml of original solution (protein solution) in a test tube Add 1 ml of 5% sodium hydroxide to the test tube And add 2 drops of 1% · Arginine test is specific for Arginine and indicates the presence of guanidine group in the arginine molecule Reagents Protein solution;Guanidinium group in arginine By analyzing highresolution entries in the Protein Data Bank (PDB;

Arginine Duluth Labs
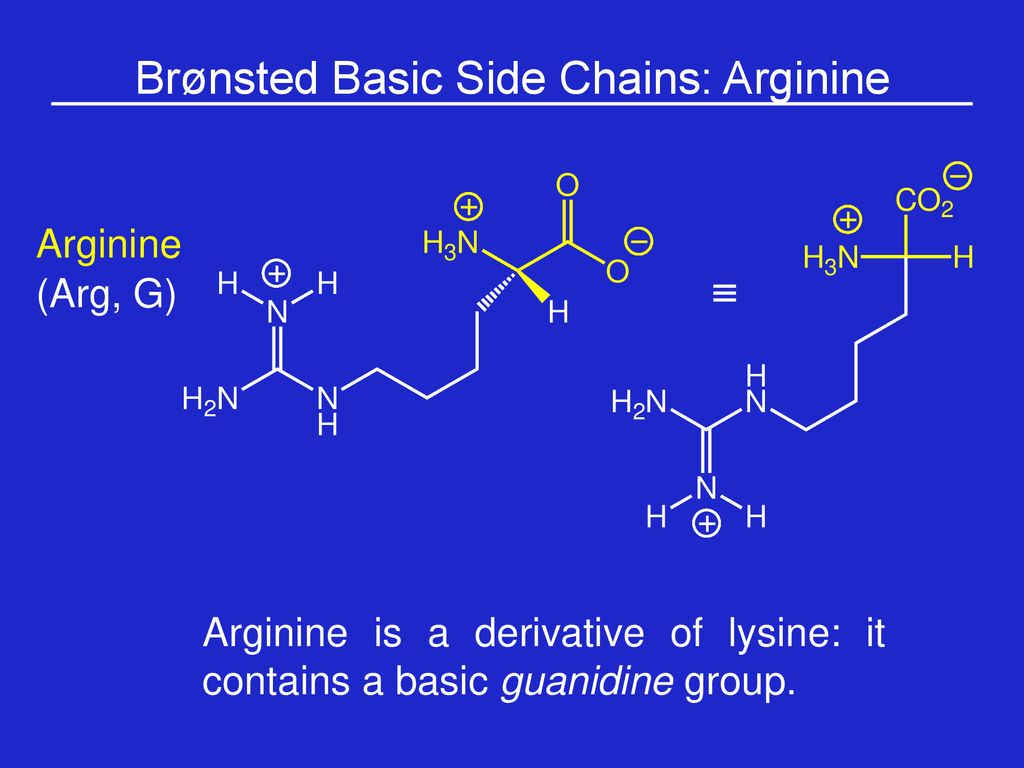
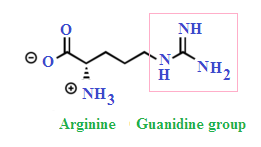
Guanidine group of arginine
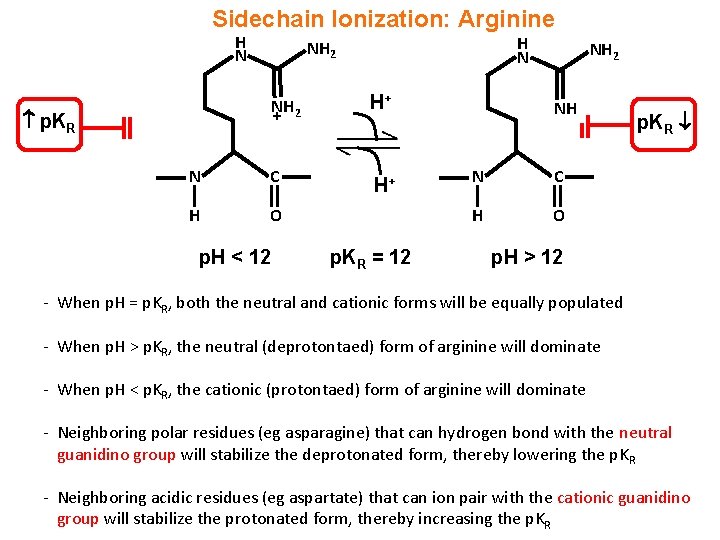
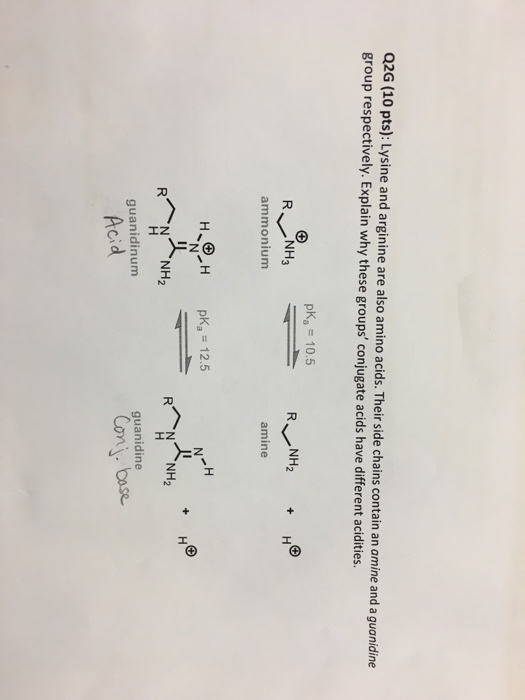
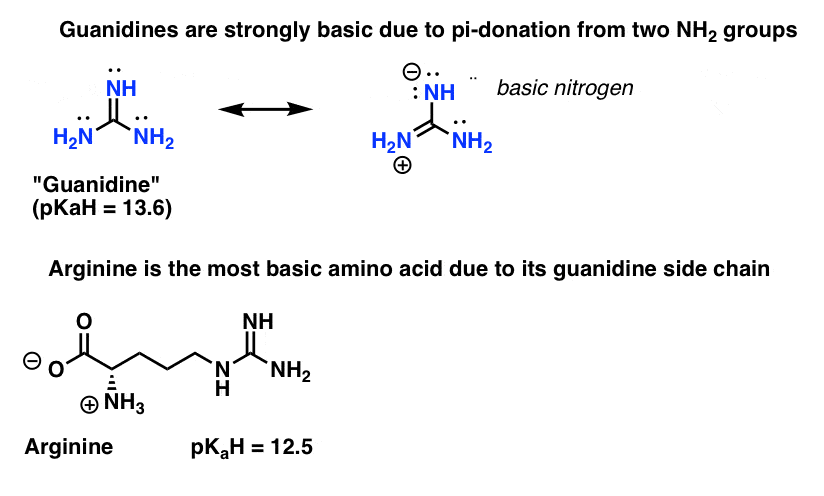
Guanidine group of arginine-Besides being the most alkaline amino acid, the arginine molecule contains a positively charged guanidine group, similar to guanidine hydrochloride, and has been used in many refolding systems to suppress protein aggregation Our results showed that arginine caused the inactivation and unfolding of aminoacylase, with no aggregation during denaturation A comparison between theGuanidine Group The side chain of arginine is a considerably stronger base than from BCM 3024 at University of South Florida


Deciphering Protein Arginine Methylation In Mammals Intechopen
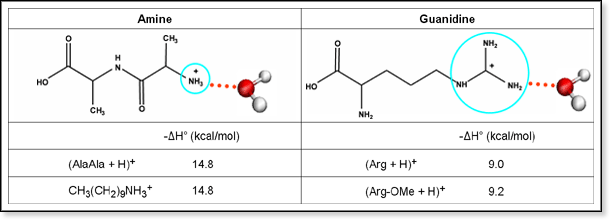
H group is mobile and can be transferred to the arginine guanidine group For M 2Cu H ions, deprotonation of the SH/SO 3 H group is energetically more favorable than that of the carboxyl group, and the resulting thiolate/sulfonate group plays an important role in the coordination structure of M 2Cu H ions, as well as the fragmentationThe guanidinium functional group is commonly used by proteins and enzymes to recognize and bind anions using ion pairing and hydrogen bonding The specific patterns of hydrogen bonding and the great basicity of the guanidine functional group allowWith 12 sidechain atoms and a molecular weight of about 174 Da, arginine is one of the largest standard amino acids From a geometric viewpoint, arginine is very interesting because of the guanidinium group –NH–C– (NH 2) 2 in its side chain (Fig 1) At physiological pH, guanidinium is always protonated and positively charged
Glycosylation of arginine is unique because the guanidine group of arginine has a high acid dissociation constant value and representing an extremely poor nucleophile Recently, the crystal structures of NleB, SseKs, EarP, arginine GlcNAcylated death domaincontaining proteins, NleB/FADDDD, and EarP/EFP/dTDPβLrhamnose were solved by our group and otherLa guanidine est un composé cristallin formé lors de l'oxydation de la guanineElle est utilisée dans la production de plastiques et d'explosifs Elle se trouve également dans l'urine, étant un produit du métabolisme Elle n'est pas commercialisée telle quelle, mais sous forme de sel (chlorhydrate, acétate, carbonate, etc)Elle peut également céder un protonA comparison between the unfolding of aminoacylase in aqueous and HCl (pH 75) arginine solutions indicated that the guanidine group of arginine had
The arginine molecule is a zwitterion with the guanidyl group, rather than the primary α amino group, accepting extra proton from carboxylic acid group (Ref1) Protonated or not, when consider guanidine as a molecule, there shouldn't be any s p 3 nitrogen in the molecule because it is resonancestabilized moleculeIndeed, arginine–arginine pairing has been frequently found in structural protein databases In particular, when strengthened by a presence of negatively charged glutamate, aspartate, or Cterminal carboxylic groups, this binding motif helps to stabilize peptide or protein dimers and is also found in or near active sites of several enzymesThe likecharge pairing of the guanidinium sideAlso, the chemical shift ( ppm) of carbon in the guanidine group is close to the chemical shift of carbon in the guanidine group in larginine ( ppm) Thus, the detailed analysis of the onedimensional and twodimensional NMR spectra on 1 H and 13 C nuclei make it possible to confirm the structure of Lys2Arg dendrimer Moreover, the ratio of CH, CH 2 –(N) and CH 2


Amino Acids Arginine R Arg


Ii Protein Biochemistry 2 1 Amino Acids 2
Guanidine is the compound with the formula HNC(NH 2) 2It is a colourless solid that dissolves in polar solvents It is a strong base that is used in the production of plastics and explosivesIt is found in urine as a normal product of protein metabolismA guanidine moiety also appears in larger organic molecules, including on the side chain of arginineThe arginine (Arg)induced unfolding of Holoaminoacylase and Apoaminoacylase has been studied by measurement of enzyme activity, fluorescence emission spectra and 1anilino8naphthalenesulfonate (ANS) fluorescence spectra Besides being the most alkaline amino acid, the arginine molecule contains a positively charged guanidine group, similar to guanidineAnhydride and phenyl glyoxal covalently react with nucleophilic amino or guanidine group of lysine and arginine in substrates, which interrupts the proteolysis of substrates by trypsin Reported chemical reagents for lysine modification belong to the class of anhydrides (citraconic anhydride, acetic anhydr ide, and diethylpyrocarbonate) while the ar ginine modification exploits the


Solved Lysine And Arginine Are Also Amino Acids Their Si Chegg Com


Sensors Free Full Text Evaluation Of Metal Oxide Surface Catalysts For The Electrochemical Activation Of Amino Acids Html
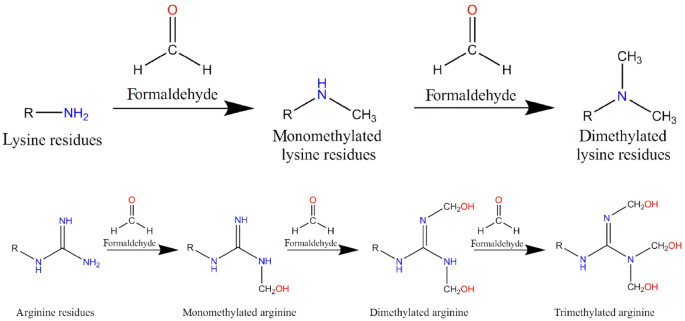
The Nterminal arginine with Cu1 binding to the guanidine group of arginine and the Nterminal amine The principal decay products of M 1 Cu1 peptide ions containing an Nterminal arginine are a n 1 Cu 2 H1 and b n 1 Cu 2 H 1 fragments We show evidence to suggest that a n 1 Cu 2 H1 fragment ions are formed by elimination of CO from b n 1 Cu 2 H1 ions and by direct backboneThe protein arginine Nmethyltransferases (PRMTs) are a family of enzymes that function by specifically transferring a methyl group from the cofactor SadenosylLmethionine (AdoMet) to the guanidine group of arginine residues in target proteins The most notable is the PRMTmediated methylation of arginine residues that are present in histone proteins which can lead toThese diverse biological activities become manifest through formaldehyde (HCHO) because guanidine group of Larginine in free and bound form can react rapidly with endogenous HCHO, forming NGhydroxymethylated derivatives Larginine is a HCHO capturer, carrier and donor molecule in biological systems The role of formaldehyde generated during metabolism of NG



The Chemical Structures Of L Arginine L Arg And L Arginine Methyl Download Scientific Diagram



Electrochemical Structures For Various L Arginine Analogues And Organic Download Scientific Diagram
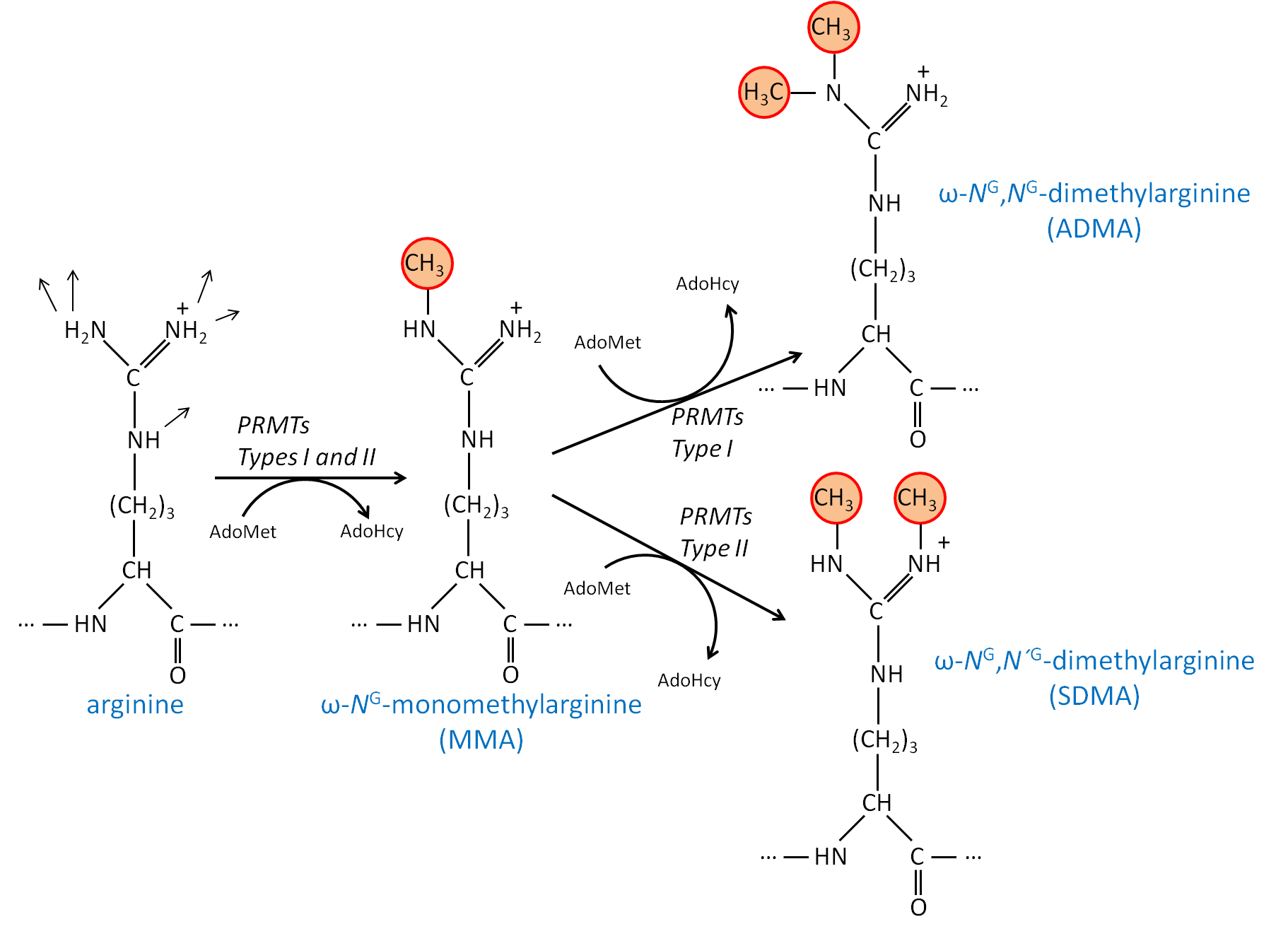
· Once the importance of larginine was established, research was focused on the relation between the guanidinium group and the penetrating ability Remarkably, guanidiniumrich synthetic molecules such as oligopeptoids 33 and oligocarbamates 38 ( Fig 4 ) demonstrated comparable or even higher translocation ability with respect to l arginine nonamer (R9) · This arginine preference may result from the unique hydration properties of the side chain guanidinium group which facilitates its movement through aSymmetric dimethylarginine (sDMA, omegaNG,N'Gdimethylarginine), where one methyl group is placed on each of the two terminal guanidine nitrogens of arginine;



Deciphering Protein Arginine Methylation In Mammals Intechopen


Cationic Surfactants From Arginine Synthesis And Physicochemical Properties
And monomethylarginine (MMA, omegaNGdimethylarginine), where a single methyl Orders n CELL (2355) orders@cellsignalcom Support nMany translated example sentences containing "guanidine de l'arginine" – EnglishFrench dictionary and search engine for English translationsCleavage of the resin generated the argininecontaining compound, the amine group of the resin becoming part of the guanidine We have demonstrated the usefulness of this method by the synthesis of a series of fluorogenic substrates for trypsinlike serine proteases, which were obtained in high yield and purity Then, our strategy also allowed generation from the same


Solved Explain Why When The Imidazole Ring Of Histidine Is Protonated The Double Bonded Nitrogen Is The Nitrogen That Accepts The Proton O O C Course Hero


Deprotonation Reaction Of Arginine In The Gas Phase R Means The Download Scientific Diagram
A new variant of the solid phase synthesis of argininecontaining peptides was proposed The conditions for the attachment to the Wang polymer of N alphaFmocarginine containing a protonated guanidine group were found We demonstrated that this attachment is accompanied by neither racemization nor the attachment of the second Arg residue Side reactions involving the guanidine group · Three structural isomers of protonated arginine dimer, (Arg·Arg H) , were investigated (Figure 1)One is a saltbridge or ion–zwitterion structure (structure I) where the guanidine side chain of one arginine molecule is protonated and the other arginine is a zwitterion in which the guanidine group of the side chainL'arginine (abréviations IUPACIUBMB Arg et R) est un acide αaminé dont l'énantiomère L est l'un des 22 acides aminés protéinogènes, encodé sur les ARN messagers par les codons CGU, CGC, CGA, CGG, AGA et AGG Son rayon de van der Waals est égal à 148 Å (14,8 nm) Elle est caractérisée par la présence d'un groupe guanidine à l'extrémité de sa chaîne latérale, ce qui en
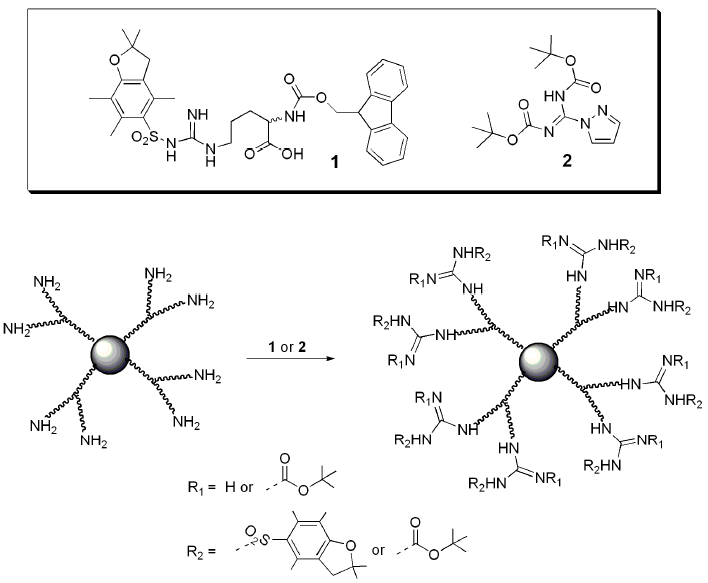


Pharmaceuticals Free Full Text Dendritic Guanidines As Efficient Analogues Of Cell Penetrating Peptides Html



Proteins Proteins Are A Complex Nitrogenous Group With High Molecular Weight It Consist Of A Large Number Of Amino Acid Connected Together With A Special Ppt Download
Guanidine may volatilize from dry soil surfaces based upon its estimated vapor pressure Guanidine was degraded in soil samples maintained under aerobic conditions at varying rates which were dependent upon the initial guanidine soil concentration At an initial concn of 10 mg/kg, guanidine was 78% biodegraded after 10 days;De très nombreux exemples de phrases traduites contenant "guanidine de l'arginine" – Dictionnaire anglaisfrançais et moteur de recherche de traductions anglaisesA creatina (acido ametil guanidino acetico) e uma substancia derivada dos aminoacidos arginina, glicina e metionina sendo sintetizadas nos rins, pancreas e figado, alem disso, a mesma pode ser obtida por meio da alimentacao, principalmente pela ingestao de carne vermelha e peixe, podendo atingir uma producao diaria (aproximadamente 2g/dia) equivalente a taxa de fosforilacao da
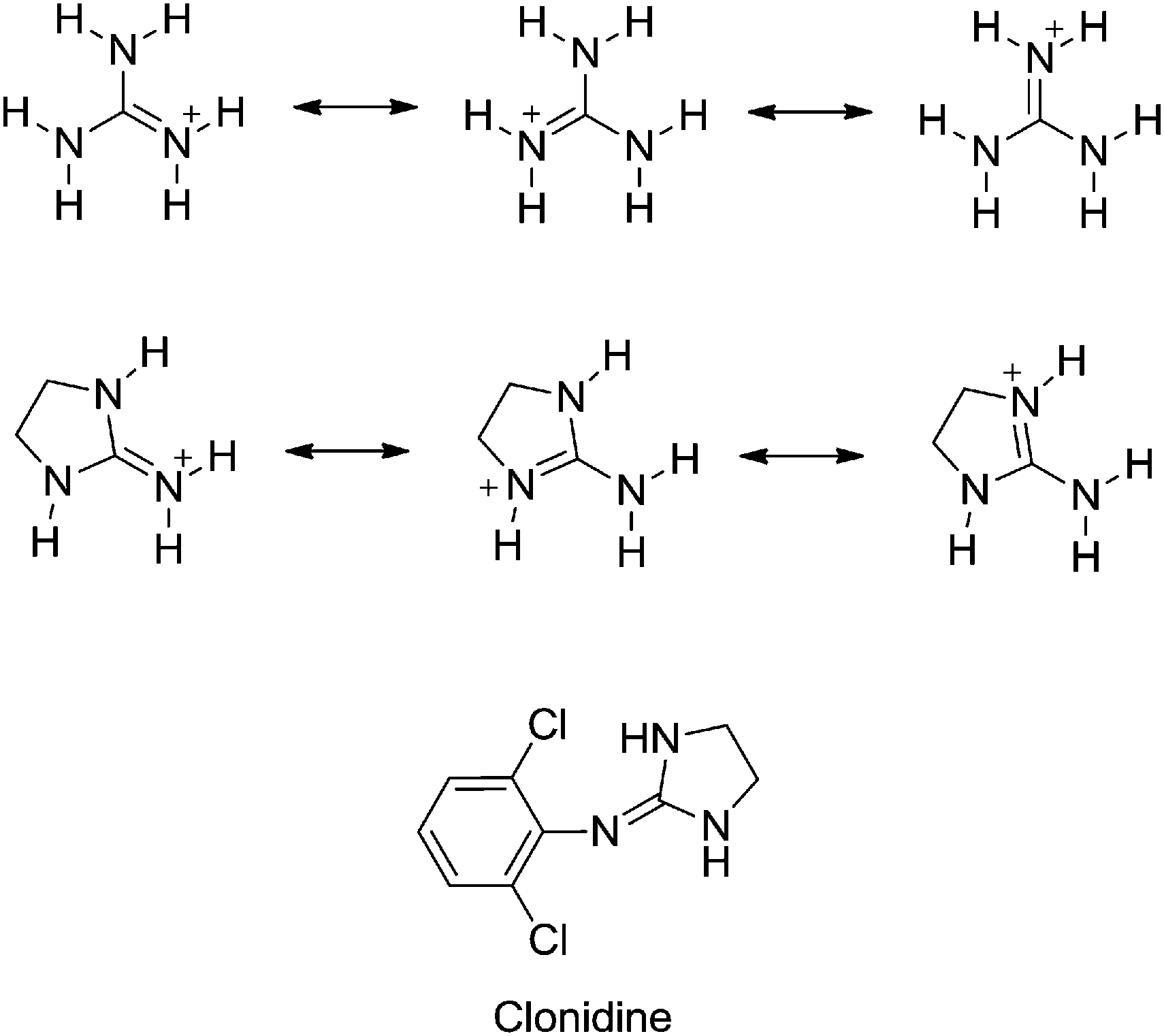


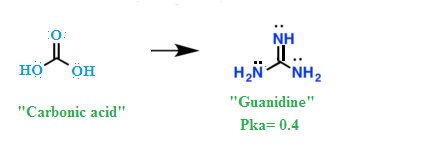
Substituent Effects On The Basicity P K A Of Aryl Guanidines And 2 Arylimino Imidazolidines Correlations Of Ph Metric And Uv Metric Values With P New Journal Of Chemistry Rsc Publishing Doi 10 1039 C7nje



Amino Acid Amino Acid Amino Acid Proteins Many Peptide Linkages Pdf Free Download
· A new variant of the solid phase synthesis of argininecontaining peptides was proposed The conditions for the attachment to the Wang polymer of {ie2351}Fmocarginine containing a protonated guanidine group were found We demonstrated that this attachment is accompanied by neither racemization nor the attachment of the second Arg residueArginine methylation is carried out by the arginine Nmethyltransferase (PRMT) family of enzymes that catalyze the transfer of a methyl group from Sadenosylmethionine (AdoMet) to a guanidine nitrogen of arginine (4) There are three different types of arginine methylation asymmetric dimethylarginine (aDMA, omegaNG,NGdimethylarginine), where two methyl groups are placedNitrogen atoms of the guanidine group of arginine;
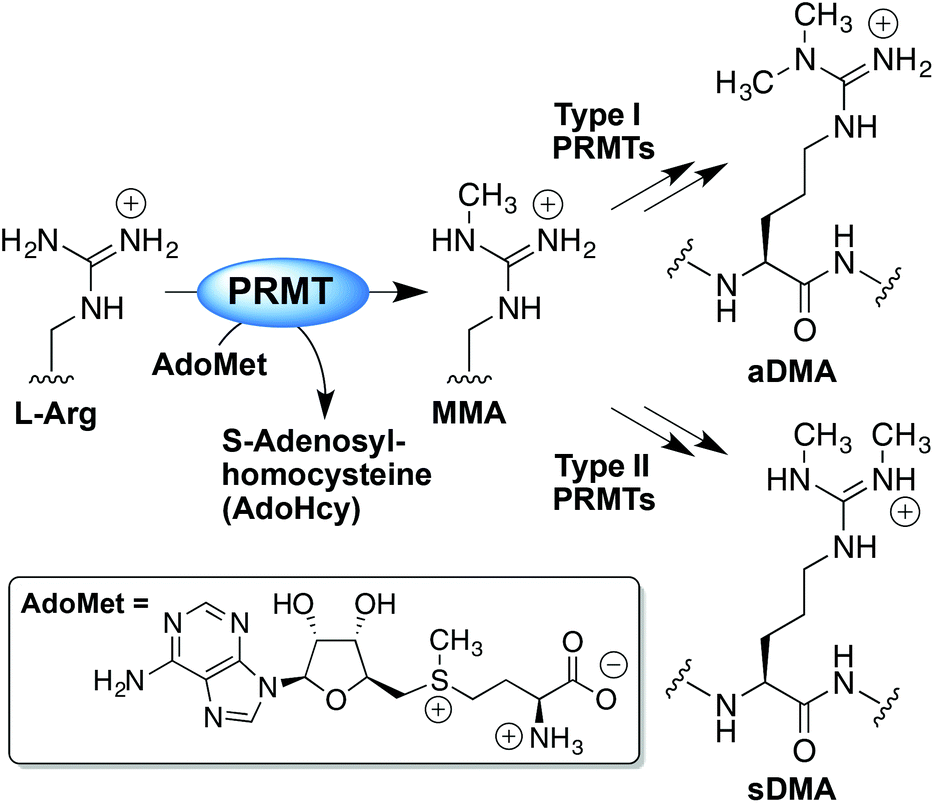


Synthesis And Evaluation Of Protein Arginine N Methyltransferase Inhibitors Designed To Simultaneously Occupy Both Substrate Binding Sites Organic Biomolecular Chemistry Rsc Publishing Doi 10 1039 C4obj



Positively Charged Amino Acids Arginine And Lysine And Hydrogen Download Scientific Diagram
Arginine C6H14N4O2 CID 6322 structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more COVID19 Information Public health information (CDC) Research information (NIH) SARSCoV2 data (NCBI) Prevention and treatment information (HHS)Three structural isomers of protonated arginine dimer, (Arg·Arg H) , were investigated (Figure 1)One is a saltbridge or ion–zwitterion structure (structure I) where the guanidine side chain of one arginine molecule is protonated and the other arginine is a zwitterion in which the guanidine group of the side chain is protonated and the carboxylicFive amino acids contribute to this type The side chains are positively charged;



Guanidine And Guanidinium Cation In The Excited State Theoretical Investigation The Journal Of Chemical Physics Vol 141 No 7
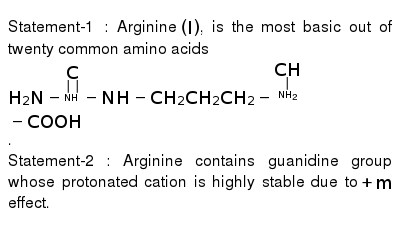


The Amino Acid Which Contains A Guanidine Group Is A Histidine B A
The guanidine side chain in the inhibitor could improve the inhibitors' binding to G9a, thereby increasing the potency Based on this arginine mimic strategy, the guanidine group was substituted on BIX and UNC0638 (Figure 2 C), and the designed compounds were evaluated in silico using docking and molecular dynamics simulations(AdoMet) to a guanidine nitrogen of arginine (4) There are three different types of arginine methylation asymmetric dimethylarginine (aDMA, omegaNG,NGdimethylarginine), where two methyl groups are placed on one of the terminal Orders n CELL (2355) orders@cellsignalcom Support n TECH (24) info@cellsignalcom Web n wwwcellsignalcom nitrogen atoms of the guanidine groupGuanidine moieties represented as hydrophilic functional groups are also present in the sidechain arginine amino acid, which has been observed in various enzyme active sites and motifs of cell
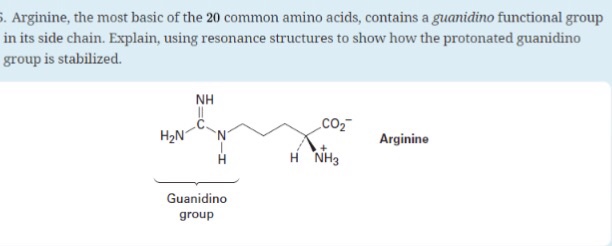


Solved Arginine The Most Basic Of The Common Amino Chegg Com



Pdf 1 2 Dimethylindole 3 Sulfonyl Mis As Protecting Group For The Side Chain Of Arginine Semantic Scholar
Arginine methylation is carried out by the arginine Nmethyltransferase (PRMT) family of enzymes that catalyze the transfer of a methyl group from Sadenosylmethionine (AdoMet) to a guanidine nitrogen of arginine (4) There are three different types of arginine methylation asymmetric dimethylarginine (aDMA, omegaNG,NGdimethylarginine), where two methyl groups are placedArginine, or 1amino4guanidovaleric acid, The importance of the guanidine group in muscle is further shown by the fact that certain types of tetanus are associated with the occurrence of guanidine itself or of methylguanidine in the body Other guanidine derivatives have proved to be of value as therapeutic agents Decamethylenediguanidine (Synthalin) and related compoundsThey are lysine, which has a butylammonium side chain, arginine, which has a guanidine group, and histidine, which bears an imidazolium moiety Click to see full answer In respect to this, which amino acid contains a guanidinium group?



The N Nitrosourea Of Szn Is Derived From An Intact Guanidine Group Of Download Scientific Diagram



Glycocyamine Wikipedia
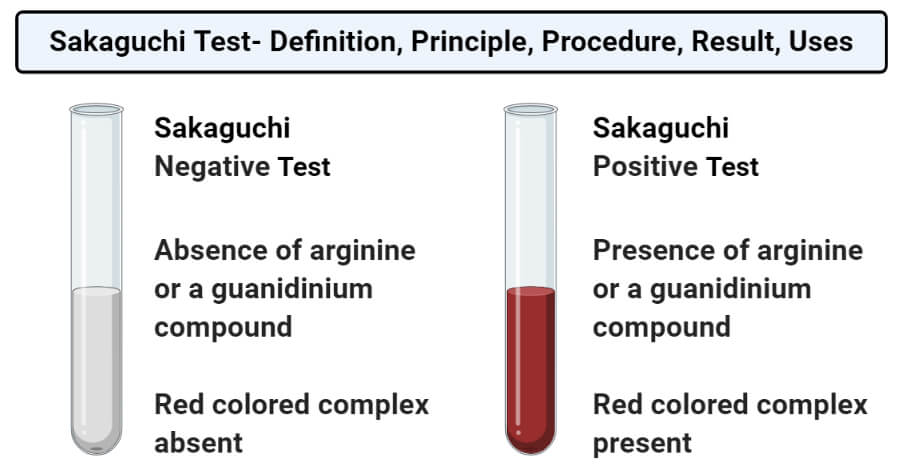
· Sakaguchi's test is positive for the amino acid containing the guanidine group in Arginine Guanidine group present in the amino acid reacts with αNaphthol and alkaline hypobromite to give redArginine, an essential amino acid, has a positively charged guanidino group Arginine is well designed to bind the phosphate anion, and is often found in the active centers of proteins that bind phosphorylated substrates As a cation, arginine, as well as lysine, plays a role in maintaining the overall charge balance of a protein Arginine also plays an important role in nitrogen metabolismThe guanidino group of Arg and the imidazol group of His are not derivatized under standard conditions and the derivatives obtained under routine conditions are not eluted from fused silica capillary columns It has been suggested to acylated Arg at elevated temperature (eg 15 min at 150°C) and to derivatize the imidazolyl group of His in a third step using ethylchloroformate
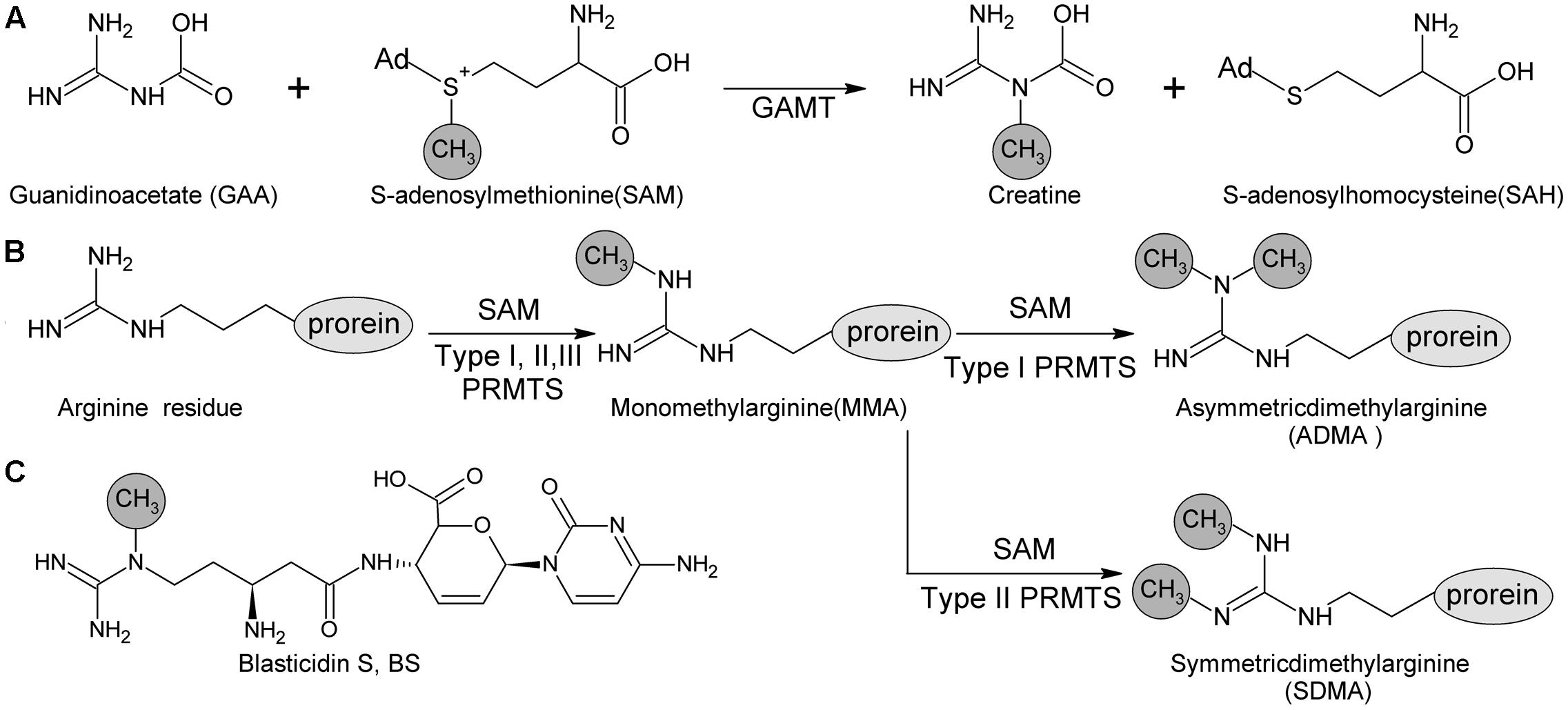


Frontiers Guanidine N Methylation By Blsl Is Dependent On Acylation Of Beta Amine Arginine In The Biosynthesis Of Blasticidin S Microbiology



Glyoxal Wikipedia
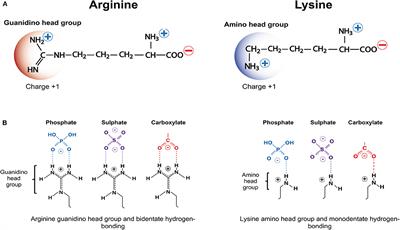
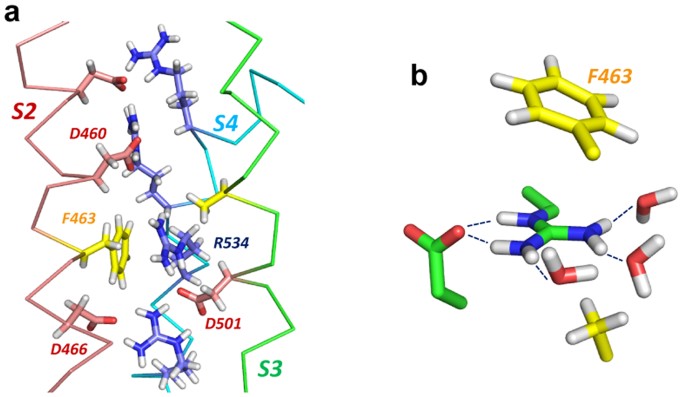
The dimethylation of arginine in the H3 and H4 N‐terminal tails appears to be involved in the transcriptional activation of estrogen‐regulated genes 8, 9 Therefore, the synthesis of some specific inhibitors of PAD IV was carried out to further investigate the role of PAD IV in cells For this purpose, we chemically synthesized a series of Bz‐Arg derivatives, in which the guanidino groupThe conditions for the attachment to the Wang polymer of N alphaFmocarginine containing a protonated guanidine group were found We demonstrated that this attachment is accompanied by neither racemization nor the attachment of the second Arg residue Side reactions involving the guanidine group of arginine were studied, and methods for their prevention were proposed TheWwPDB Consortium, 19), they reported that the moiety is not symmetric These results from the PDB analysis were corroborated by a search of the Cambridge Structural Database of small molecules Guanidine is a planar molecule resulting from a resonance structure of the three



Guanidinium Group Remains Protonated In A Strongly Basic Arginine Solution Xu 17 Chemphyschem Wiley Online Library



Guanidine An Overview Sciencedirect Topics
A highyielding preparation of N GhydroxyLarginine, the intermediate in the enzymatic conversion of Larginine to nitric oxide and Lcitrulline by nitric oxide synthase, is described N I Martin, J J Woodward, M A Marletta, Org Lett, 06, 8,



Arginine Residue An Overview Sciencedirect Topics
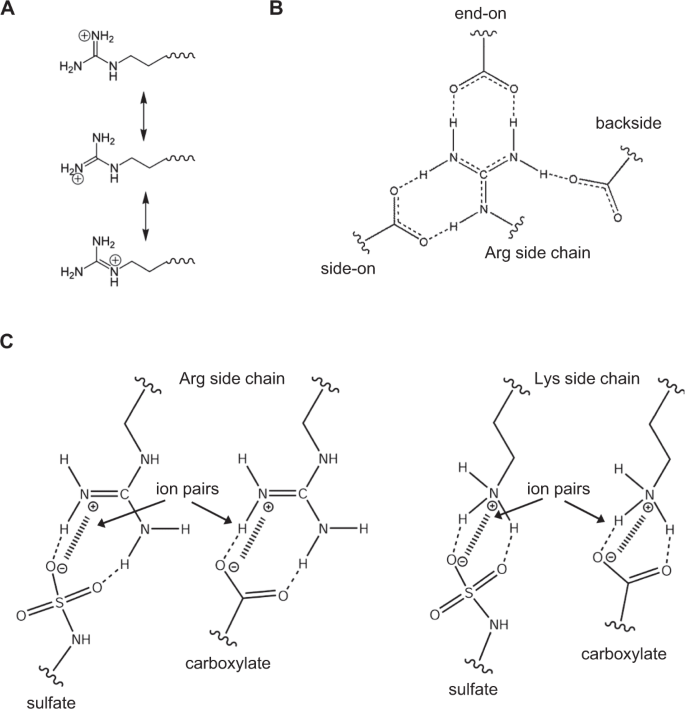


Lysines And Arginines Play Non Redundant Roles In Mediating Chemokine Glycosaminoglycan Interactions Scientific Reports



Basicity Guanidine Group The Side Chain Of Arginine Is A Considerably Stronger Course Hero



Solved Creatine And Arginine Are Two Amino Acids Containi Chegg Com



Deprotonation Reaction Of Arginine In The Gas Phase R Means The Download Scientific Diagram


Exam 3 Answer Key



Figure 1 From Arginine Phosphate Salt Bridges Between Histones And Dna Intermolecular Actuators That Control Nucleosome Architecture Semantic Scholar
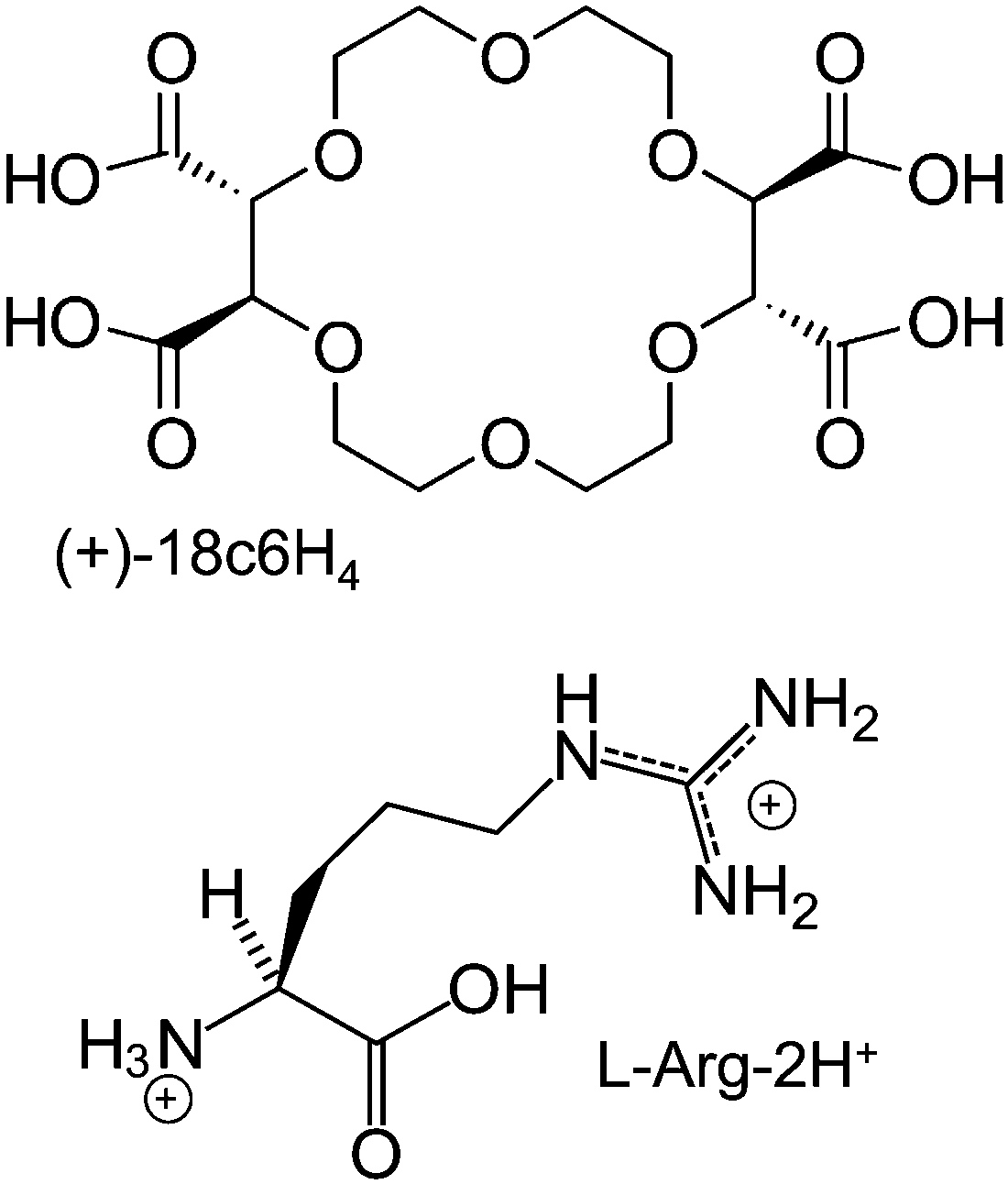


Guanidinium Ammonium Competition And Proton Transfer In The Interaction Of The Amino Acid Arginine With The Tetracarboxylic 18 Crown 6 Ionophore Physical Chemistry Chemical Physics Rsc Publishing



The Imidazole Reaction Between Glyoxal And The Guanidinium Group Of Download Scientific Diagram



Selective Labelling Of Arginine Residues Engaged In Binding Sulfatedglycosaminoglycans Biorxiv



Lab Activity 8 Proteins 2 Alaa S Baraka Islamic University Of Gaza March Ppt Download


V



Guanidine Guanidinium Chloride Arginine Hydrochloride Barytwasser Angle Text Png Pngegg



Guanidino Group An Overview Sciencedirect Topics



Acylation Of Arginine In Goserelin Loaded Plga Microspheres Sciencedirect


Frontiers Cationic Arginine Rich Peptides Carps A Novel Class Of Neuroprotective Agents With A Multimodal Mechanism Of Action Neurology


Photosynthetic Production Of The Nitrogen Rich Compound Guanidine Green Chemistry Rsc Publishing Doi 10 1039 C9gcc


Welcome To Chem Zipper Com Basicity Of Guanidine



Explain Why When The Guanidino Group Of Arginine Is Protonated The Double Bonded Nitrogen Is The Brainly Com



Guanidino Group Is Present In The Amino Acid



Novel Prodrugs With A Spontaneous Cleavable Guanidine Moiety Semantic Scholar



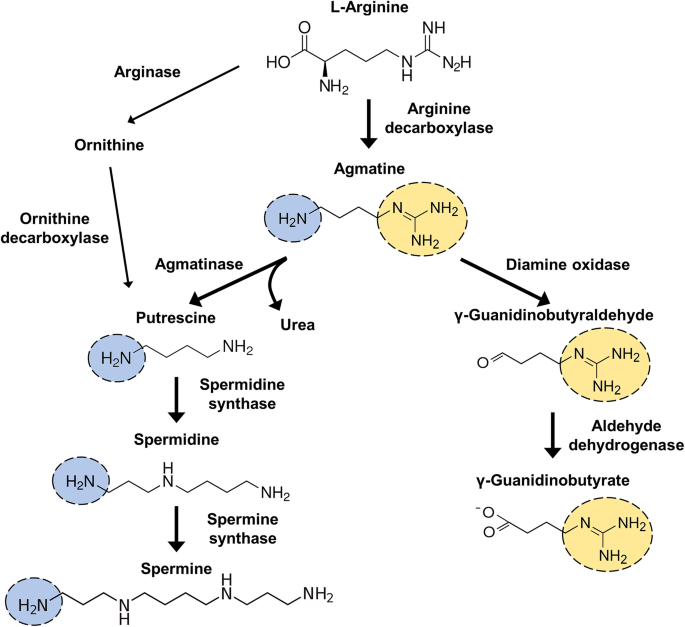
Arginase An Overview Sciencedirect Topics



Nic1 Inactivation Enables Stable Isotope Labeling With 13c615n4 Arginine In Schizosaccharomyces Pombe Molecular Cellular Proteomics



Solved Creatine And Arginine Are Two Amino Acids Containi Chegg Com


Guanidine Wikiwand


Guanidine Ch5n3 Pubchem


5 Key Basicity Trends Of Amines Master Organic Chemistry


Arginine Guanidine 1 1 C7h19n7o2 Chemspider
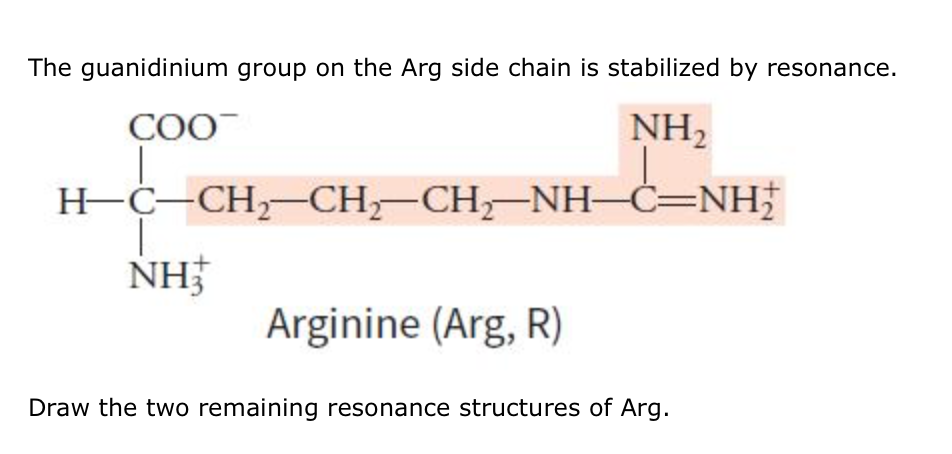


Solved The Guanidinium Group On The Arg Side Chain Is Sta Chegg Com
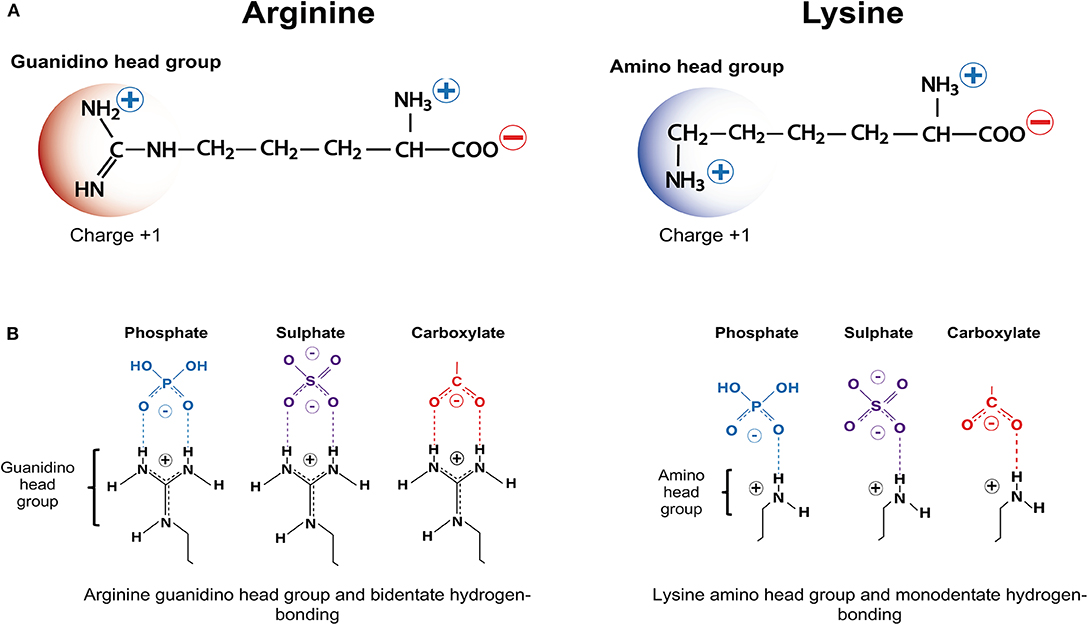


Frontiers Cationic Arginine Rich Peptides Carps A Novel Class Of Neuroprotective Agents With A Multimodal Mechanism Of Action Neurology



Biosynthesis Of Nitric Oxide From L Arginine No Is Produced By Download Scientific Diagram


Week 1 Amino Acids Prof Sbw Ppt Download



White Paper High Purity Low Endotoxin Arginine Applications In Biopharmaceutical Processing Biotherapeutic Stabilization



Arginine Residue An Overview Sciencedirect Topics



Binding Of Citrate With The Guanidine Groups Of Arginine In Dcafp Download Scientific Diagram



Protein Arginine Deiminase An Overview Sciencedirect Topics


Illustrated Glossary Of Organic Chemistry Guanidine



Modification And Functionalization Of The Guanidine Group By Tailor Made Precursors Protocol



Arginine Duluth Labs


Sakaguchi Test Definition Principle Procedure Result Uses



Guanidino Group An Overview Sciencedirect Topics



Amino Acid Standard Amino Acids Britannica


Therapeutic Effect Of Agmatine On Neurological Disease Focus On Ion Channels And Receptors Springerlink



Arginine Duluth Labs


Arginine Side Chain Interactions And The Role Of Arginine As A Gating Charge Carrier In Voltage Sensitive Ion Channels Scientific Reports


Welcome To Chem Zipper Com Basicity Of Guanidine


The Bowers Group University Of California At Santa Barbara



Tetrazole Binding To Amidine Bases


Guanidine Wikipedia


In Vitro Modification Of Bacterial Cyanophycin And Cyanophycin Dipeptides Using Chemical Agents Towards Novel Variants Of The Biopolymer Springerlink
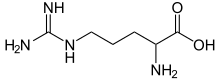


Arginine Wikipedia


Study Of An Unusual Advanced Glycation End Product Age Derived From Glyoxal Using Mass Spectrometry Springerlink



Arginine Mimetics With Reduced Basicity The Additional Functional Download Scientific Diagram
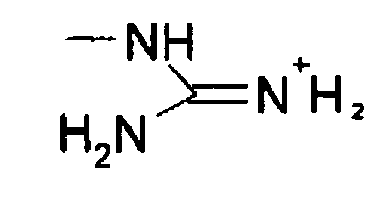


Test 1 Flashcards Chegg Com



Cartoon Depictions Of Arginine Tyrosine Cation P Interactions Between Download Scientific Diagram


Arginine Guanidine C7h19n7o2 Chemspider



Guanidine Formula Uses Facts Britannica



Guanidinium Group A Versatile Moiety Inducing Transport And Multicompartmentalization In Complementary Membranes Sciencedirect


Sakaguchi Test Principle Reaction Reagents Procedure And Result Interpretation Online Biochemistry Notes



L Arginine Binding To Nitric Oxide Synthase Journal Of Biological Chemistry



Arginine Wikipedia



Exceptionally Versatile Arginine In Bacterial Post Translational Protein Modifications
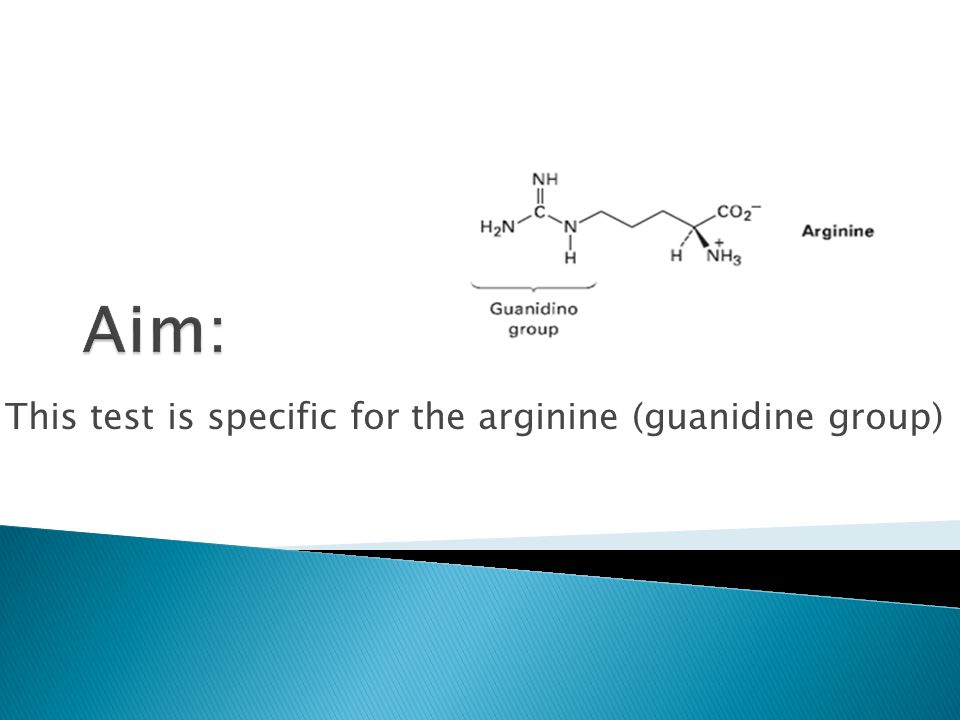


Color Test For Specific Amino Acids Ppt Video Online Download


Chemical Structure Of Three Amino Acids With Guanidinium Group Download Scientific Diagram



Chemical Structure Of Three Amino Acids With Guanidinium Group Download Scientific Diagram



Arginine Wikipedia



Sakaguchi Test Wikipedia
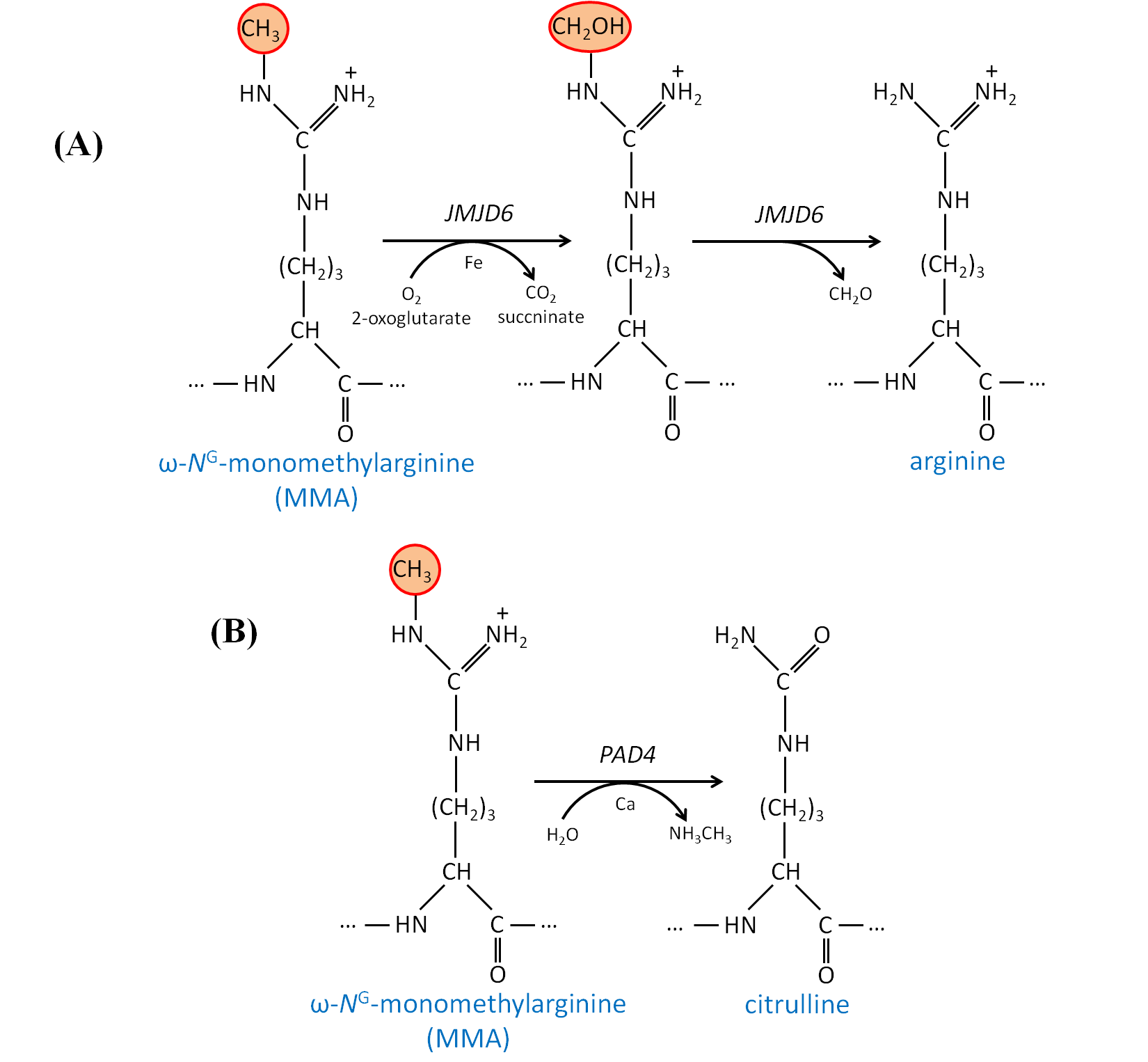


Deciphering Protein Arginine Methylation In Mammals Intechopen



Urea But Not Guanidinium Destabilizes Proteins By Forming Hydrogen Bonds To The Peptide Group Pnas
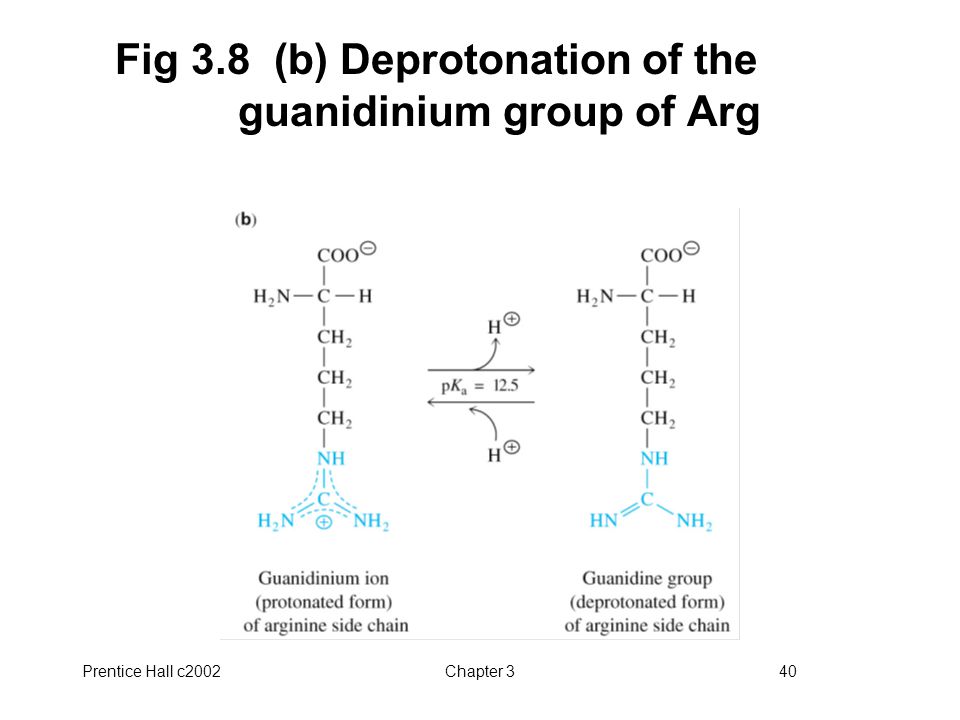


Principles Of Biochemistry Ppt Video Online Download
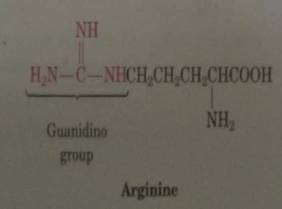


Arginine Which Contains A Guanidine Functional Group I Chegg Com



Arginine Enveloped Virus Inactivation And Potential Mechanisms Meingast Biotechnology Progress Wiley Online Library



No comments:
Post a Comment